Today, we will be exploring the primary concepts of decaffeination and examining how traditional and Swiss approaches to this process differ from one another.
A fascinating tale tells that Ludwig Roselius, in 1903, unintentionally discovered a process to decaffeinate coffee. While it is unknown if this was on purpose or accidental, he eventually sold the technique to an American firm that used it until they found out its hazardous effects - benzene can still stay after decaffeination and presented health risks.
With over two hundred different ways of decaffeination, naturally decaffeinated coffee still raises questions. In this article, you will gain an understanding of what the process is and how traditional and Swiss methods differ. You'll also learn about the various ways to get your fix without all that caffeine!
Why is caffeine removed from coffee?
Caffeine, an alkaloid naturally occurring in plants such as coffee, tea, and cocoa and mate can protect them from leaf-eating insects and attract pollinators. When consumed by animals like us humans it stimulates the central nervous system to drive up our pulse rate for a feeling of invigoration; one that just cannot be replicated any other way!
An occasional cup of coffee can be a great way to jumpstart your morning, so long as you are healthy. Yet those with cardiovascular concerns, expecting mothers, and young children must remain cautious; even small doses of caffeine could hurt their well-being.
The process of decaffeination involves extracting caffeine from green coffee beans, an action that requires two primary steps.
- Eliminate caffeine from your diet, but be sure to omit any aromatic or flavoring additives as well.
- Ensure that all traces of decaffeination substances have been removed from the product.
To begin the decaffeination process, unroasted beans must first be submerged in heated water to open them up. The rest of the procedure varies based on which method you utilize: some use solvents while others rely on carbon dioxide and organic concentrates. As a result, this will drastically affect both the quality and taste of your coffee. Let’s compare traditional Swiss decaffeination methods to examine what kind of coffee we can expect from solvent treatments compared with natural organic concentrate alternatives!
When it comes to the comparison between traditional and Swiss methods of decaffeination, the former was discovered first but later discarded due to its use of benzene. This hazardous chemical stripped coffee beans of caffeine yet left a portion behind in the grains that worsened liver function when consumed. Luckily, with an innovative solution like Switzerland's process, you can enjoy your cup-a-joe without any worries about potential health risks - all while still avoiding a jolt from too much caffeine!
To evaluate the difference between the traditional and Swiss brewing methods, we chose to compare those that use a solvent (like Ludwig Roselius) with those that rely exclusively on water and a common carbon filter.
The European, conventional, or direct process known as the traditional way is by far the most widely used decaffeination method.
Decaffeination follows a precise process: initially, coffee is steamed for 30 minutes. Afterward, they are exposed to methylene chloride or ethyl acetate - solvents that extract the caffeine from the grains - for 10 hours. Finally, any remaining residues of those substances are removed by soaking them for an additional 10 hours.
The coffee is decaffeinated through the natural process of using ethyl acetate, instead of methylene chloride. This compound is extracted from fruits and cane sugar to achieve 99.9% caffeine-free coffee without compromising its flavor. By opting for this type of decaffeination method, we can guarantee that our customers will enjoy a smooth cup with all the taste and none of the buzz!
The Swiss Water Process also referred to as the water or non-chemical decaffeination method, is a complicated yet eco-friendly approach for coffee caffeine removal. It may be pricier than other methods, but its result and environmental impact make it worth the investment.
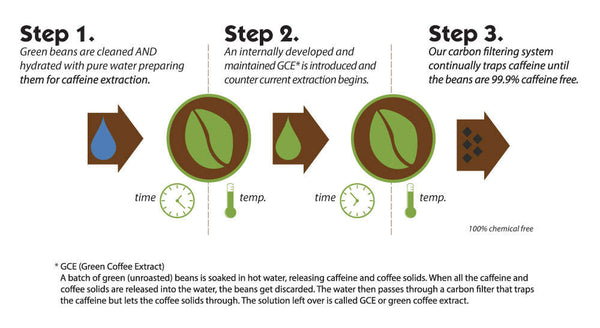
The Swiss approach to decaffeinating coffee is a straightforward process. Initially, Green Coffee Extract (GCE) is prepared from green beans. To do this, the initial batch of beans is immersed in water for some time so that all substances can be extracted including caffeine, and then passed into the water solution.
By allowing the extract to pass through a carbon filter system, caffeine molecules are caught in the filter while flavoring elements remain behind and become part of the final product. As such, you get an extract that has all of its flavor but none of its stimulant properties; an ideal choice for those who don't need or want their coffee with caffeine.
Then, the following batch of grains is soaked in the prepared extract. The ingredients of both grain and extract strive for equilibrium once they come into contact with each other due to the equivalent flavor elements present in them. However, since caffeine solely exists within the grains, it passes over to the brew so that a balance can be preserved; whereas all aroma and taste components remain intact in their original form inside those seeds.
Unfortunately, it is impossible to eliminate caffeine from coffee beans in just one cycle of steeping. To solve this issue, the extracted liquid then undergoes a process with carbon filters that removes all traces of caffeine before being put back into the beans multiple times until their count drops down to an incredibly low 0.01%.
The Swiss method provides you with an unparalleled way to decaffeinate coffee while retaining all the desired flavor and aroma of the beans. Not only is this high-quality taste superior, but it's also safe for your heart as no chemicals remain in the grains after processing - up to 99.9% of caffeine can be removed!
Video about the swiss decaffeination method
When it comes to decaffeination, the Swiss method reigns supreme. Not only does it produce a richer flavor and better quality coffee than using solvents typically employed in traditional methods, but its animation-backed video makes understanding this complex process simple! Unfortunately, though, that superiority doesn't come cheap - you'll likely pay more for coffee de-caffeinated with the Swiss method than those processed via other means; yet when weighed against an improved taste experience, is it worth making such a small sacrifice?
What to know
Mass producers generally lean toward the traditional decaffeination technique since it is faster and more cost-effective. Consuming store-bought, decaffeinated coffee often means that caffeine has been eliminated from it through solvents, none of which pose a hazard to our health - not like benzene used to be! The only downside is that the Swiss process keeps marginally more flavor elements intact.

